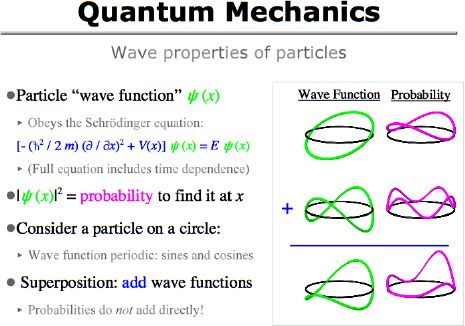
There is an awful lot to say about quantum mechanics, and I hardly even have time to touch on it here. The essential point is that the state of a particle (or any other system) is described by its "wave function". The behavior of that wave function (and hence of the particle) is determined by the Schrödinger equation with an appropriate potential energy function V(x).
It is significant that the wave function takes complex values, not just real ones. When two wave functions are added in a superposition, those complex numbers are what allow them to "interfere" with each other in interesting ways.
In common examples where V(x) is constant, the Schrodinger equation is just the spatial part of a classic wave equation. In this special case, the equation has sines and cosines (or linear combinations of them) as solutions. Some of those solutions are illustrated in the first column of the picture here: the circumference of the circle determines the allowed "wavelengths" for the sines and cosines. (Of course, I've only drawn the real part of the wave function in each case.)
Up to my research page.
Up to my professional page.
My personal site is also available.
Any questions or comments? Write to me: jensens@alma.edu
Copyright © 2004 by Steuard Jensen.